 Multiple Choice Questions
Multiple Choice QuestionsThe following are some statements related to VA group hydrides,
(I) Reducing property increases from NH3 to BiH3
(II) Tendency to donate lone pair decreases from NH3 to BiH3.
(III) Thermal stability of hydrides decreases from NH3 to BiH3.
(IV). Bond angle of hydrides decreases from NH3 to BiH3.
The correct statements are:
I, II, III and IV
I, III and IV
L, II and IV
I and IV
The type of bonds present in sulphuric anhydride are
3σ and three p-d
3σ and one p-p and two p-d
2σ and three p-d
2σ and two p-d
B.
3σ and one p-p and two p-d
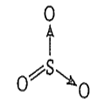
Sulphuric anhydride is SO3 and it consists of 3σ, one p-p and p-d bonds. Its structure is as follows-
Which pair of oxyacids of phosphorus contains 'P-H' bonds?
H3PO4, H3PO3
H3PO5, H4P2O7
H3PO3, H3PO2
H3PO2, HPO3
Which one of the following is formed apart from sodium chloride when chlorine reacts with hot concentrated sodium hydroxide?
NaOCl
NaClO3
NaClO2
NaClO4
Helium mixed with oxygen is used in the treatment of
beri beri
burning feet
joints burning
asthma
Phosgene is formed slowly from which one of the following on exposure to air and sunlight?
CHCl3
H3CCl
H3COH
C2H5Cl
The volume in mL of 0.1 M solution of NaOH required to completely neutralise 100 mL of 0.3 M solution of H3PO3 is
60
600
300
30
