 Multiple Choice Questions
Multiple Choice QuestionsExtraction of chlorine from brine solution based on
Oxidation
acidification
chlorination
reduction
Which chlorine atom is more electronegative in the following ?
CH3-Cl
CH3-CH2-Cl
H--Cl
CH3-CH2--Cl
Which one of the following noble gas has an unusual property of diffusing through the materials such as rubber, glass or plastic?
Kr
Ne
Ar
He
Select wrong chemical reaction among the following.
8NH3 + 3Cl2 → 6NH4Cl + N2
2Ca(OH)2 + Cl2 → Ca(OCl)2 + CaCl2 + 2H2O
2NaOH + Cl2 → 2NaCl + H2 + O2
MnO2 + 4HCl → MnCl2 + Cl2 + 2H2O
In the reaction :
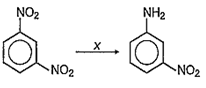
X is -
SiC
H2SO4
KMnO4
Fe/ HCl
D.
Fe/ HCl
Reduction of NO2 group to NH2 group is taking place in reaction-
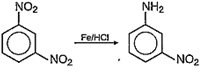
Fluorine exhibits an oxidation state of only -1 because:
it can readily accept an electron
it is very strongly electronegative
it is a non-metal
it belong to halogen family
Which among the following factors is the most important in making fluorine the strongest oxidizing agent ?
Electron affinity
Ionization enthalpy
Hydration enthalpy
Bond dissociation energy
