 Multiple Choice Questions
Multiple Choice QuestionsIn trimeta-phosphate ion, the number of 0 atom P-O-P bonds and unit negative charges are respectively:
6,6,3
3,6,3
9,3,3
9,6,3
C.
9,3,3
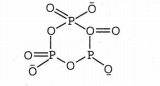
Trimetaphosphate ion is as follow:
Which of the following is the laboratory method for synthesis of nitric oxide?
Reaction of zinc with cold and dilute HNO3
Reaction of zinc with concentrated HNO3
Reaction of copper with cold and dilute HNO3
Heating NH4NO3
Which one of the following phosphorous compound is used during electrode position of metal on rubber and for making pesticides?
Phosphorus pentachloride (PCl5)
Phosphorus (Ill) oxide (P4O8)
Phosphorus M oxide (P4O10)
Phosphorus trichloride (PCl3)
Reduction potential of some ions are given below:
Arrange them in decreasing order of oxidising power.
Ions Reduction : Cl I Br
Potential Ev = 1.19 V Ev = 1.741
Potential Ev / V
Cl > I < Br
I > Br < Cl
Br < I < Cl
Br < Cl < I
Conc. H2SO4 displaces hydrogen chloride from chlorides because:
it is stronger acid
sulphates are less soluble than chlorides
sulphates are more soluble than chlorides
HCl is a gas while H2SO4 is a liquid
Liquid flow from a higher to a lower level.Which of the following liquids can climb up the walls of the glass vessel in which it is placed?
Mercury
Liquid N2
Liquid He (II)
Water
A greenish yellow gas reacts with alkali metal hydroxide to form a halate which can be used in fire works and safety matches. The gas and halate respectively are :
B2 , KBrO3
Cl2 , KClO3
I2 , NaIO3
Cl2 , NaClO3
When plants and animals decay, the organic nitrogen is converted into inorganic nitrogen. The inorganic nitrogen is in the form of :
ammonia
elements of nitrogen
nitrates
nitrides
