 Multiple Choice Questions
Multiple Choice QuestionsSulphur does not exist as S2 molecule because
it is less electronegative
it is not able to constitute bonds
it has ability to exhibit catenation
of tendency to show variable oxidation states
Oxidation number of sulphur in Caro's acid is
+6
+4
+8
+7
A.
+6
Caro's acid is H2SO5. It has a peroxide linkage, so, oxidation of S is
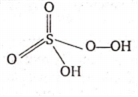
Let the oxidation state of S is x
H2SO5 (one peroxide bond)
+ 2 + x + 3(- 2) + 1(- 2) = 0
2+ x - 6- 2 = 0
x - 6 =0
x = 6
Liquor ammonia is
ammonium hydroxide
liquified ammonia gas
concentrated solution of NH3 in water
a solution of NH3 in alcohol
The number of moles of hydrogen molecules required to produce 20 moles of ammonia through Haber's process is :
20
30
40
10
Which is the correct thermal stability order for H2E (E = O, S, Se, Te and Po)?
H2O < H2S < H2Se < H2Te < H2Po
H2Po < H2Te < H2Se < H2S < H2S
H2Se < H2Te <H2Po < H2O < H2S
H2S < H2O < H2Se < H2Te <H2Po
