 Multiple Choice Questions
Multiple Choice QuestionsThe temperature of the cold junction of thermocouple is 0°C and the temperature of hot junction is To C. The emf is E = 16T - 0.04T2 µV. The inversion temperature Tf is
300o C
200o C
500o C
400o C
The temperature of a gas is raised from 27° C to 927° C. The root mean square speed
gets halved
gets doubled
is times the earlier value
remains the same
Two different isotherms representing the relationship between pressure p and volume V at a given temperature of the same ideal gas are shown for masses m1 and m2 , then
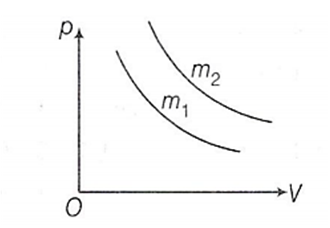
Nothing can be predicted
m1 < m2
m1 = m2
m1 > m2
1 mole of H2 gas is contained in a box of volume V = 1.00 m3 at T = 300 K. The gas is heated to a temperature of T = 3000 K and the gas gets converted to a gas of hydrogen atoms. The final pressure would be (considering all gases to be ideal)
same as the pressure initially
2 times the pressure initially
10 times the pressure initially
20 times the pressure initially
Assertion: Vibrational energy of diatomic molecule corresponding to each degree of freedom is kB T.
Reason: For every molecule, vibrational degree of freedom is 2.
If both assertion and reason are true and reason is the correct explanation of assertion.
If both assertion and reason are true but reason is not correct explanation of assertion.
If assertion is true but reason is false.
If both assertion and reason are false.
Pressure versus temperature graph of an ideal gas is as shown in figure. Density of the gas at point A is ρ0 Density at point B will be
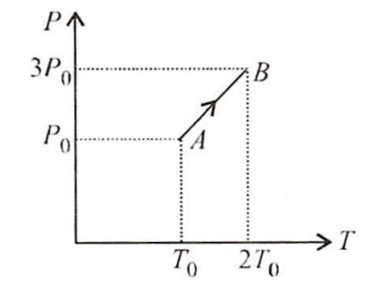
ρ0
ρ0
ρ0
2ρ0
B.
ρ0
Density ( ρ )
ρ =
P is the pressure of the gas
V is the volume of the gas
R is the universal gas constant
T is absolute temperature of gas
ρ ∝
and
∴ ρB = ρA
ρB = ρ0
Two balloons are filled, on with pure He gas and other by air, respectively. If the pressure and temperature of these balloons arc same then the number of molecules per unit volume is
more in the He filled balloon
same in both balloons
more in air filled balloon
in the ratio of 1 : 4
Assertion: The root mean square and most probable speeds of the molecules in a gas are the same.
Reason: The Maxwell distribution for the speed of molecules in a gas is symmetrical.
If both assertion and reason are true and reason is the correct explanation of the assertion
If both assertion and reason are true but reason is not the correct explanation of the assertion
If assertion is true, but reason is false
Both assertion and reason are false statements
vrms , vav and vmp are root mean square, average and most probable speeds of molecules of a gas obeying Maxwellian velocity distribution. Which of the following statements is correct?
vrms < vav < vmp
vrms > vav > vmp
vmp > vrms > vav
vmp > vrms > vav
