 Multiple Choice Questions
Multiple Choice QuestionsAssertion: It is not possible for a system, unaided by an external agency to transfer heat from a body at lower temperature to another body at a higher temperature.
Reason: It is not possible to violate the second law of thermodynamics
If both assertion and reason are true and reason is the correct explanation of assertion.
If both assertion and reason are true but reason is not the correct explanation of assertion.
If assertion is true but reason is false.
If both assertion and reason are false.
In a cyclic process, work done by the system is
zero
more than the heat given to the system
equal to heat given to the system
independent of heat given to system
Ina cylinder their are 60 g Ne and 64 g O2, If pressure of mixture of gases in cylinder is 30 bar then in this cylinder partial pressure of O2 is (in bar)
30
20
15
12
A gas mixture contain one mole O2 gas and one mole He gas. Find the ratio of specific heat at constant pressure to that at constant volume of the gaseous mixture
2
1.5
2.5
4
One mole of oxygen of volume 1 litre at 4 atm pressure lo attains 1 atm pressure by result of isothermal expansion. Find work done by the gas.
≈ 155 J
≈ 206 J
≈ 355 J
≈ 552 J
Which of the following is not a state function?
Work-done in adiabatic process.
Work done in isothermal process.
Heat at constant pressure.
Heat at constant volume.
The molar specific heats of an ideal gas at constant pressure and volume are denoted by CP and CV respectively. If and R is then CV universal gas constant, then CV is equal to
γR
Conversion of water to steam is accompanied by which process?
Adiabatic
Isothermal
Isochoric
Cyclic
An ideal gas is taken through the cycle A → B → C → A, as shown in figure. If the net heat supplied to the gas in the cycle is 5 J the work done by the gas in the process A → B is

2 J
3 J
4 J
5 J
B.
3 J
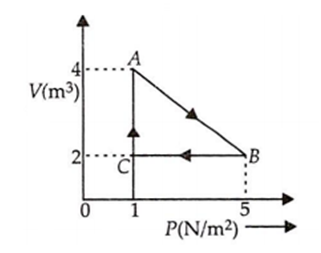
Process B → C occurs at constant volume
Hence WBC = 0
Process C → A occurs at constant process.
Hence the work done in the process is
WCA = P ΔV
= (1) ( VA - VC )
WCA = (1) (4 - 2)
WCA = 2J
For cyclic process is
Δu = 0
According to the first law of thermodynamics
ΔQ = ΔV + Δu
ΔQ = ΔW
= WAB + WBC +WCA
5 = WAB + 0 + 2
Since net heat supplied 5 J
WAB = 3 J
