 Multiple Choice Questions
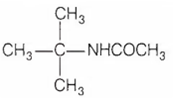
Multiple Choice QuestionsAn organic compound X having molecular formula C3H11N reacts with p-toluene sulphonyl chloride to form a compound Y that is soluble in aqueous KOH. Compound X is optically active and reacts with acety lchloride to form compound Z. Identify the compound Z
CH3CH2CH2CH2NHCOCH3
CH3CH2CH(CH3)NHCOCH3
CH3(CH3)CHCH2NHCOCH3
Diethyl amine when treated with nitrous acid yields
diethyl ammonium nitrite
ethyl alcohol
N-nitroso diethyl amine
triethyl ammonium nitrite
The amine 'A' when treated with nitrous acid gives yellow oily substance. The amine A is
triethylamine
trimethylamine
aniline
methylphenylamine
D.
methylphenylamine
Secondary amines when treated with nitrous acid give yellow oily substance. So, amine 'A' is methylphenyl amine. Reaction of nitrous acid with aliphatic amines,
Liberation of N2 gas + alcohol formation
R-NH2 + HNO2 → R-N+≡NCl- ROH + N2 + HCl
Reaction of nitrous acid with aromatic amines
Liberation of N2 gas + Phenol formation
PhNH2 + HNO2 + HCl Ph-N≡NCl- → PhOH + HCl + N2
Reaction of nitrous acid with 2° amines: With HNO2, they give nitroso amines, which being insoluble in dilute acids separate as yellow oily compound.
R2NH + HONO → R2-N-N=O
N-nitroso dialkyl amine (yellow oil)
Reaction of nitrous acid with 3° amines : Aliphatic 3° amines on reaction with HONO formsoluble nitrite salts, while aromatic 3° amines form green coloured p -nitroso amines.

In case of substituted aniline the group which decreases the basic strength is
-OCH3
-CH3
-NH2
-C6H5
Which of the following statement(s) is/are incorrect in case of Hofmann bromamide degradation?
Reaction is useful for decreasing length of carbon chain by one carbon atom
It gives tertiary amine
It gives primary amine
Aqueous or alco. KOH is used with bromine
The amine, which reacts with p-toluenesulphonyl chloride to give a clear solution, which on acidification gives insoluble compound is
C2H5NH2
(C2H5)2NH
(C2H5)3N
CH3NHC2H5
Which one of the following can be prepared by Gabriel phthalimide synthesis?
Aniline
o- toluidine
Benzylamine
N-methylethanamine
0ne mole of hydrazine (N2H4) loses 10 moles of electrons in a reaction to form a new compound X. Assuming that all the nitrogen atoms in hydrazine appear in the new compound, what is the oxidation state of nitrogen in X? (Note - There is no change in the oxidation state of hydrogen in the reaction).
-1
-3
+3
+5
Which one of the following is used as a test for aliphatic primary amines?
Tollen's test
Fehling's test
lsocyanide test
Azo dye test
When methanamine is treated with benzoyl chloride, the major product is
N-phenylethanamide
N-methylbenzamide
benzanilide
acetophenone
