 Multiple Choice Questions
Multiple Choice QuestionsAn element X belongs to fourth period and fifteenth group of the periodic table. Which of the following statements is true?
It has completely filled s-orbital and a partially filled d-orbital.
It has completely filled s-and p -orbitals and a partially filled d-orbital.
It has completely filled s- and p-orbitals and a half filled d-orbital.
It has a half filled p -orbital and completely filled s-and d-orbitals.
Of the following molecules, which have shape similar to CO2?
HgCl2
SnCl2
C2H2
NO2
A.
HgCl2
C.
C2H2
HgCl2 and C2H2 both show linear shape as of CO2.
(·: both have zero lone pair of electrons)
(a) HgCl2
(b) 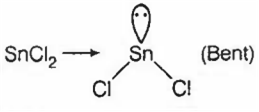
(c)
(d) 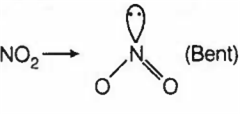
The number of lone pairs of electrons on the central atoms of H2O, SnCl2, PCl3 and XeF2 respectively are
2, 1, 1, 3
2, 2, 1, 3
3, 1, 1, 2
2, 1, 2, 3
The correct order of decreasing H-C-H angle in the following molecule is

I> II> III
II> I> III
III> II> I
I> III> II
The correct order of decreasing length of the bond as indicated by the arrow in the following structures is

I >II > III
II > I > III
III > II > I
I > III > II
In SOCl2, the Cl-S-Cl and Cl-S O bond angles are
130° and 115°
106° and 96°
107° and 108°
96° and 106°
In O2 and H2O2 , the O-O bond lengths are 1.21 and 1.48 Å respectively. In ozone, the average O-O bond length is
1.28 Å
1.18 Å
1.44 Å
1.52 Å
In diborane, the number of electrons that accounts for bonding in the bridges is
six
two
eight
four
