 Multiple Choice Questions
Multiple Choice QuestionsAn element X belongs to fourth period and fifteenth group of the periodic table. Which of the following statements is true?
It has completely filled s-orbital and a partially filled d-orbital.
It has completely filled s-and p -orbitals and a partially filled d-orbital.
It has completely filled s- and p-orbitals and a half filled d-orbital.
It has a half filled p -orbital and completely filled s-and d-orbitals.
The number of lone pairs of electrons on the central atoms of H2O, SnCl2, PCl3 and XeF2 respectively are
2, 1, 1, 3
2, 2, 1, 3
3, 1, 1, 2
2, 1, 2, 3
A.
2, 1, 1, 3
The number of lone pairs of electrons on the central atoms of H2O, SnCl2, PCl3 and XeF2 are 2, 1, 1 and 3 respectively.
![]() Number of lone pairs = 2
Number of lone pairs = 2
Cl--Cl Number of lone pairs = 1
 Number of lone pair = 1
Number of lone pair = 1
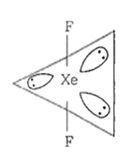 Number of lone pairs = 3
Number of lone pairs = 3
The correct order of decreasing H-C-H angle in the following molecule is

I> II> III
II> I> III
III> II> I
I> III> II
The correct order of decreasing length of the bond as indicated by the arrow in the following structures is

I >II > III
II > I > III
III > II > I
I > III > II
In SOCl2, the Cl-S-Cl and Cl-S O bond angles are
130° and 115°
106° and 96°
107° and 108°
96° and 106°
In O2 and H2O2 , the O-O bond lengths are 1.21 and 1.48 Å respectively. In ozone, the average O-O bond length is
1.28 Å
1.18 Å
1.44 Å
1.52 Å
In diborane, the number of electrons that accounts for bonding in the bridges is
six
two
eight
four
