 Multiple Choice Questions
Multiple Choice QuestionsThe rate of a reaction is primarily determined by the slowest step .This step is called :
rate determining step
reaction rate
activation step
None of the above
For a reaction, r= k(CH3COCH3)3/2 then unit of rate of reaction and rate constant respectively is
mol L-1 s-1, mol-1/2 L1/2 s-1
mol-1 L-1 s-1, mol-1/2 L-1/2 s-1
mol L-1 s-1 mol+1/2L1/2s-1
mol L s, mol+1/2 L 1/2 s
If t1/2 vs is a straight line graph then determine the order of reaction.
Zero order
First order
Second order
Third order
Energy of activation of forward reaction for an endothermic process is 50 kJ. If enthalpy change for forward reaction is 20 kJ then enthalpy change for backward reaction will be
30 kJ
20 kJ
70 kJ
50 kJ
A.
30 kJ
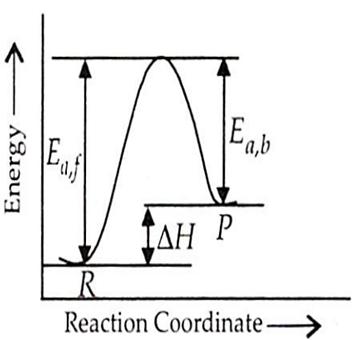
For endothermic reaction,
Ea,b = Ea,f -
= 50 kJ - 20 kJ = 30 kJ
Assertion : Rate of reaction doubles when concentration of reactant is doubled if it is a first order reaction.
Reason : Rate constant also doubles.
If both assertion and reason are true and reason is the correct explanation of assertion.
If both assertion and reason are true but reason is not the correct explanation of assertion.
If assertion is true but reason is false.
If both assertion and reason are false.
A radioactive substance decays th of it original value in 2h. The half-life of the substance is :
1 h
30 min
20 min
15 min
The chemical reaction 2O3 3O2 proceeds as :
(I) O3 O2 + O (fast)
(II) O + O3 2O2 (slow)
The rate expression should be :
r = k[O3]2
r = k[O3]2 [O2]-1
r = k[O3] [O2]
None of these
For a 1st order reaction if concentration is doubled then rate of reaction becomes
doubles
half
four times
remains same
The first order rate constant for dissociation of N2O5 is 6.2 x 10-4 s-1 .The half-life period (in s) of this dissociation will be :
1117.7
111.7
223.4
160.9
