 Multiple Choice Questions
Multiple Choice QuestionsPick out the correct statement with respect
[Mn(CN)6]3–
It is sp3d2 hybridised and octahedral
It is sp3d2 hybridised and tetrahedral
It is d2sp3 hybridised and octahedral
It is d2sp3 hybridised and octahedral
Match the metal ions given in Column I with the spin magnetic moments of the ions given in Column II and assign the correct code :
| Column I | Column II | ||
| a. | Co3+ | i. | |
| b. | Cr3+ | ii. | |
| c. | Fe3+ | iii. | |
| d. | Ni2+ | iv. | |
| v. | |||
| a | b | c | d |
| iv | v | ii | i |
| a | b | c | d |
| i | ii | iii | iv |
| a | b | c | d |
| iii | v | i | ii |
| a | b | c | d |
| iv | i | ii | iii |
Which one of the following ions exhibits d-d transition and paramagnetism as well?
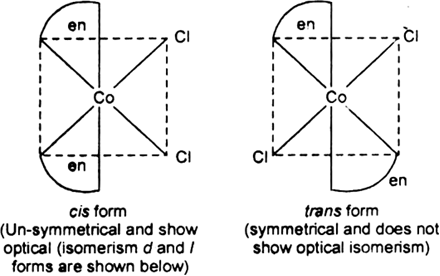
The type of isomerism shown by the complex [CoCl2(en)2] is
Geometrical isomerism
Coordination isomerism
Linkage isomerism
Ionization isomerism
A.
Geometrical isomerism
In the given complex, the CN of Co is 6, and the complex has octahedral geometry.
The geometry and magnetic behaviour of the complex [Ni(CO)4] are
Square planar geometry and diamagnetic
Tetrahedral geometry and diamagnetic
Tetrahedral geometry and paramagnetic
Square planar geometry and paramagnetic
Consider the following species:
CN+, CN–, NO and CN
Which one of these will have the highest bond order?
NO
CN-
CN
CN+
Which of the following coordination compounds would exhibit optical isomerism?
Pentamminentirocobalt (III) iodide
Tris-(ethylenediamine) cobalt (III) bromide
Trans-dicyanobis (ethylenediamine)
Diamminedinitroplatinum (II)
Sulphur reacts with chlorine in 1:2 ratio and forms X. Hydrolysis of X gives a sulphur compound Y. The hybridization of the central atom in the anion Y is
sp3
sp2
sp3d
sp
