 Multiple Choice Questions
Multiple Choice QuestionsConsider the following E° values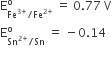
Under standard conditions the potential for the reaction
Sn(s) + 2Fe3+(aq) → 2Fe2+(aq) + Sn2+(aq) is
1.68 V
0.63 V
0.91 V
0.91 V
The standard e.m.f of a cell, involving one electron change is found to be 0.591 V at 25°C. The equilibrium constant of the reaction is (F = 96,500 C mol-1: R = 8.314 JK-1 mol-1)
1.0×101
1.0×1030
1.0×1030
1.0×1030
D.
1.0×1030
Relation between Keq and Ecell is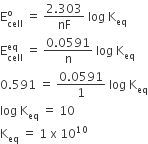
The limiting molar conductivities Λ° for NaCl, KBr and KCl are 126, 152 and 150 S cm2 mol-1 respectively. The Λ° for NaBr is
128 S cm2 mol-1
302 S cm2 mol-1
278 S cm2 mol-1
278 S cm2 mol-1
In a cell that utilises the reaction Zn(s) + 2H+ (aq) → Zn2+(aq) + H2(g) addition of H2SO4 to cathode compartment, will
lower the E and shift equilibrium to the left
increases the E and shift equilibrium to the left
increase the E and shift equilibrium to the right
increase the E and shift equilibrium to the right
The 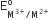 values for Cr, Mn, Fe and Co are – 0.41, +1.57, + 0.77 and +1.97 V respectively. For which one of these metals the change in oxidation state form +2 to +3 is easiest?
values for Cr, Mn, Fe and Co are – 0.41, +1.57, + 0.77 and +1.97 V respectively. For which one of these metals the change in oxidation state form +2 to +3 is easiest?
Cr
Co
Fe
Fe
How long (approximate) should water be electrolysed by passing through 100 amperes current so that the oxygen released can completely burn 27.66 g of diborane? (Atomic weight of B = 10.8 u)
1.6 hours
6.4 hours
0.8 hours
3.2 hours
During electrolysis of molten NaCl, some water was added. What will happen?
Electrolysis will stop
Hydrogen will be evolved
Some amount of caustic soda will be formed
A fire is likely
The role of fluorspar, which is added in small quantities in the electrolytic reduction of alumina dissolved in fused cryolite is
as a catalyst
to make fused mixture conducting
to lower the melting temperature of the mixture
to decrease the rate of oxidation of carbon at anode
At temperature of 298K, the emf of the following electrochemical cell,
Ag (s) | Ag+ (0.1 M)|| Zn2+ (0.1 M) | Zn (s)
will be (Given, E°cell = -1.562 V)
-1.532 V
-1.503 V
1.532 V
-3.06 v
The quantity of electricity needed to separately electrolyze 1 M solution of ZnSO4 , AlCl3 and AgNO3 completely is in the ratio of
2 : 3 :1
2 : 1 : 1
2 : 1:3
2 : 2 : 1
