 Multiple Choice Questions
Multiple Choice QuestionsWhen CO2 is passed through solution of sodium aluminate, precipitate of which compound is formed?
Al(OH)3
Al2O3
Na2CO3
No ppt
Which of the following is not the correct match?
Leaching : Ag
Zone refining : Sn
Liquation : Pb
van Arkel : Zr
The purpose of smelting an ore is to :
oxidise it
reduce it
obtain an alloy
separate volatile impurities
What is the role of aniline or cresol when added in a froth floatation process?
Stabilizer
Depressant
Wetting agent
All of these
Oils can be converted into fats by :
hydrogenolysis
hydrogenation
decarboxylation
hydration
B.
hydrogenation
Oils can be converted into solid fats by the process of hydrogenation .
Hydrogenation : The vegetable oils containing glycerides of unsaturated fatty acids , undergo catalytic hydrogenation with hydrogen in presence of finely divided nickel .This results in conversion of a liquid glyceride (i.e , an oil) into solid glyceride (i.e , a fat)
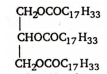 + 3H2
+ 3H2 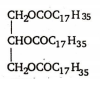 (fat)
(fat)
Carbon and CO gas are used to reduce which of the following pairs of metal oxides for extraction of metals?
FeO, SnO
SnO, ZnO
BaO, Na2O2
FeO, ZnO
