 Multiple Choice Questions
Multiple Choice QuestionsFind the product for
CH3CH2-O-CH2-CH2-O-CH2-C6H5+ HI (excess)
HO-CH2CH2OH, C6H5CH2-I, CH3CH2-I
C6H5CH2-OH, CH3CH2-I, I-CH2CH2-OH
I-CH2CH2-I, C6H5CH2-I, CH3CH2-OH
HO-CH2CH2-OH, C6H5CH2-I, CH3CH2-OH
SN2 reaction readily occurs in
CH3CH2-O-CH3
CH3-C(CH3)2-O-CH3
CH2=CH-CH2-O-CH3
Ph-CH2-O-CH2-CH3
An alkyl halide reacts with alcoholic ammonia in a sealed tube , the product formed will be :
a primary amine
a secondary amine
a tertiary amine
a mixture of all the three
D.
a mixture of all the three
When an alkyl halide reacts with alcoholic ammonia in a sealed tube then a mixture of primary , secondary and tertiary amines is formed .
RX + NH3 + HX
RNH2 + XR + HX
R2NH + XR + HX
The catalyst used in the preparation of an alkyl chloride by the action of dry HCl on an alcohol is :
anhydrous AlCl3
Fecl3
anhydrous ZnCl2
Cu
In which of the following preparations of ether, the configuration about chiral centre is not retained?
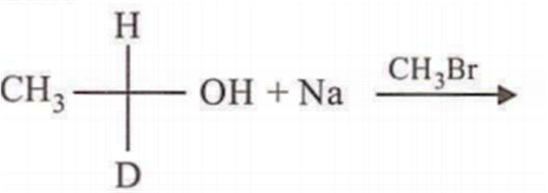
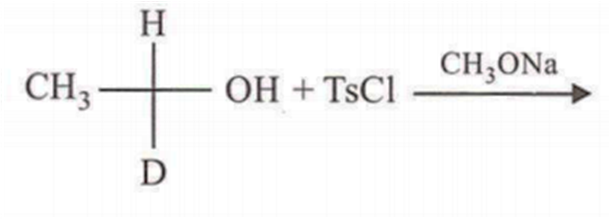

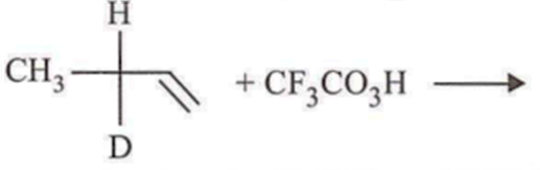
Which ofthe following compounds is not chiral?
1-Chloropentane
2-Chloropentane
1-Chloro-2-methyl pentane
3-Chloro-2-methyl pentane
