 Multiple Choice Questions
Multiple Choice QuestionsIn 2-butene, which one of the following statements is true?
C1-C2 bond is a sp3- sp3- -bond
C2- C3 bond is sp3 - sp2- -bond
C1- C2 bond is a sp3 - sp2 - -bond
C1- C2 bond is a sp2- sp2- -bond
The ease of nitration of the following three hydrocarbons follows the order

II - III ≈ I
II > III > I
III > II > I
I = III > II
B.
II > III > I
The ease of nitration of the hydrocarbons depends upon the stability of arenium ion formed.
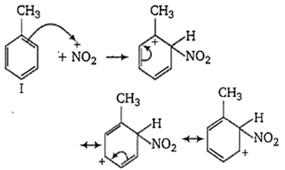
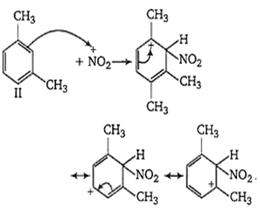
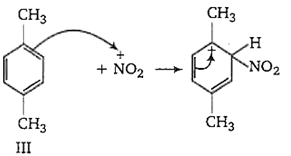

In compound II, the carbonium ion has two methyl groups capable of partially neutralising the positive charges on the ring carbon atoms.
In compound III, two methyl group has +I effect whereas in compound I, only one methyl group with +I effect is present. Thus, the stability of arenium ion and ease of nitration follows the order II > III > I.
Among the alkenes which one produces tertiary butyl alcohol on acid hydration?
CH3-CH2-CH=CH2
CH3-CH=CH-CH3
(CH3)2C=CH2
CH3-CH=CH2
Friedel-Craft's reaction using MeCl and anhydrous AlCl3 will take place most efficiently with
benzene
nitrobenzene
acetophenone
toluene
Under identical conditions, the SN1 reaction will occur most efficiently with
tert-butyl chloride
1-chlorobutane
2-methyl-1-chloropropane
2-chlorobutane
An equimolar mixture of toluene and chlorobenzene is treated with a mixture of conc.H2SO4 and cone. HNO3. Indicate the correct statement from the following.
p-nitrotoluene is formed in excess
equimolar amounts of p-nitrotoluene and p-nitrochlorobenzene are formed
p-nitrochlorobenzene is formed in excess
m-nitrochlorobenzene is formed in excess
Among the following carbocations:
Ph2C+CH2Me (I), PhCH2CH2CH+Ph (II),Ph2CHCH+Me (III) and Ph2C(Me)CH2 (IV), the order of stability is
IV > II > I > III
I > II > III > IV
II > I > IV > III
I > IV > III > II
The reaction of toluene with chlorine in presence of ferric chloride gives predominatly
benzoyl chloride
m - chlorotoluene
benzyl chloride
o- and p - chlorotoluene
In benzene, the triple bond consists of
one sp-sp sigma bond and two p-p pi bonds
two sp-sp sigma bonds and one p-p pi bond
one sp2-sp2 sigma bond, one p-p pi bond
one sp2-sp2 sigma bond, one sp2-sp2 pi bond and one p-p pi bond
