 Multiple Choice Questions
Multiple Choice QuestionsEthylene glycol is used as an antifreeze in a cold climate. Mass of ethylene glycol which should be added to 4 kg of water to prevent it form freezing at - 6° C will be : (Kf for water = 1.86 K kg mol-1, and molar mass of ethylene glycol = 62g mol-1)
804.32 g
204.30 g
400.00 g
400.00 g
A vessel at 1000 K contains CO2 with a pressure of 0.5 atm. Some of the CO2 is converted into CO on the addition of graphite. If the total pressure at equilibrium is 0.8 atm, the value of K is
1.8 atm
3 atm
0.3 atm
0.3 atm
In the context of the lanthanoids, which of the following statement is not correct?
There is a gradual decrease in the radii of the members with increasing atomic number in the series.
All the member exhibit +3 oxidation state.
Because of similar properties, the separation of lanthanoids is not easy.
Because of similar properties, the separation of lanthanoids is not easy.
If sodium sulphate is considered to be completely dissociated into cations and anions in aqueous solution, the change in freezing point of water (ΔTf), when 0.01 mol of sodium sulphate is dissolved in 1 kg of water, is (Kf = 1.86 K kg mol–1).
0.0372 K
0.0558 K
0.0744 K
0.0744 K
On mixing, heptane and octane form an ideal solution. At 373 K, the vapour pressures of the two liquid components (heptane and octane) are 105 kPa and 45 kPa respectively.Vapour pressure of the solution obtained by mixing 25.0 g of heptane and 35 g of octane will be (molar mass of heptane = 100 g mol–1 and of octane =114 g mol–1)
144.5 kPa
72.0 kPa
36.1 kPa
36.1 kPa
The freezing point of benzene decreases by 0.45°C when 0.2 g of acetic acid is added to 20 g of benzene. If acetic acid associates to form a dimer in benzene, percentage association of acetic acid in benzene will be
(Kf for benzene = 5.12 K kg mol–1)
64.6%
80.4%
74.6%
74.6%
On treatment of 100 mL of 0.1 M solution of CoCl3 . 6H2O with excess AgNO3; 1.2 × 1022 ions are precipitated. The complex is
[Co(H2O)4 Cl2]Cl.2H2O
[Co(H2O)3Cl3].3H2O
[Co(H2O)6]Cl3
[Co(H2O)6]Cl3
D.
[Co(H2O)6]Cl3

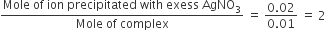
It means 2Cl– ions present in ionization sphere
∴ complex is [Co(H2O)5Cl]Cl2.H2O
1 gram of a carbonate (M2CO3) on treatment with excess HCl produces 0.01186 mole of CO2. The molar mass of M2CO3 in g mol-1 is
1186
84.3
118.6
118.6
Two liquids X and Y form an ideal solution. At 300 K, the vapour pressure of the solution containing 1 mol of X and 3 mol of Y is 550mm Hg. At the same temperature, if 1 mol of Y is further added to this solution, the vapour pressure of the solution increases by 10 mm Hg. Vapour pressure (in mmHg) of X and Y in their pure states will be respectively
200 and 300
300 and 400
400 and 600
400 and 600
A binary liquid solution is prepared by mixing n-heptane and ethanol. Which one of the following statements is correct regarding the behaviour of the solution?
The solution formed is an ideal solution
The solution is non-ideal, showing +ve deviation from Raoult’s law.
The solution is non-ideal, showing –ve deviation from Raoult’s law.
The solution is non-ideal, showing –ve deviation from Raoult’s law.
