 Multiple Choice Questions
Multiple Choice QuestionsWhich of the following is an example for heterogeneous catalysis reaction ?
Hydrolysis of aqueous sucrose solution in the presence of aqueous mineral acid
Hydrolysis of liquid in the presence of aqueous mineral acid
The disperse phase, dispersion medium and nature of colloidal solution (lyophilic or lyophobic) of 'gold sol' respectively are :
solid, solid, lyophobic
liquid, liquid, lyophobic
solid, liquid, lyophobic
solid, liquid, lyophilic
Assertion (A) : A catalyst increases the rate of a reaction.
Reason (R) : In presence of a catalyst, the activation energy of the reaction increases.
The correct answer is
Both (A) and (R) are true and (R) is the correct explanation of (A).
Both (A) and (R) are true but (R) is not the correct explanation of (A).
(A) is true, but (R) is not true
(A) is not true, but (R) is true
Which of the following is not correct?
Milk is a naturally occurring emulsion
Gold sol is a lyophilic sol
Physical adsorption decreases with rise in temperature
Chemical adsorption is unilayered
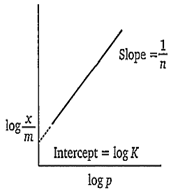
Which one of the following gives a straight line for Freundlich adsorption isotherm?
log vs log p
log
A.
log vs log p
Freundlich adsorption isotherm is as-
= Kp1/n
taking logarithms on both the sides
On plotting a graph between log x/m and log p, a straight line will be obtained.
Match the following:
| List - I | List - II |
| A. Solid dispersed in liquid | i. Emulsion |
| B. Liquid dispersed in liquid | ii. Foam |
| C. Gas dispersed in liquid | iii. Gel |
| D. Liquid dispersed in solid |
iv. Sol v. Aerosol |
The correct match is
A - iv; B - i; C - ii; D - iii
A - iii; B - i; C - v; D - ii
A - iii; B - i; C - ii; D - iv
A - iv; B - i; C - v; D - iii
Gelly is a colloidal solution of
solid in liquid
liquid in solid
liquid in liquid
solid in solid
Which of the following is the most effective in causing coagulation of ferric hydroxide solution?
KCl
KNO3
K2SO4
K3[Fe(CN)6]
Which of the following statements is not correct inrespect of chemisorption?
Highly specific adsorption
Irreversible adsorption
Multilayered adsorption
High enthalpy of adsorption
