 Multiple Choice Questions
Multiple Choice QuestionsWhich one of the following oxides of nitrogen dimerises into colourless is solid/ liquid on cooling?
N2O
NO
N2O3
NO2
Halogens exist in -1,+ 1, + 3, + 5 and 47 oxidation states. The halogen that exists only in -1 state is
F
Cl
Br
I
Among the oxyacids of phosphorus, the dibasic acid is
H3PO3
H4P2O7
H2PO2
HPO3
A.
H3PO3
H3PO3 is a dibasic acid as it contains two replaceable hydrogen atoms.
(a) 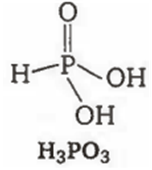
(b) 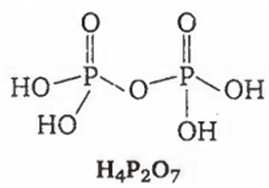
(c) 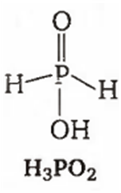
(d) 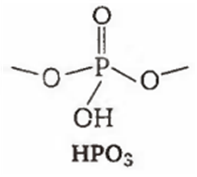
Which of the following is the correct method of preparation of methyl fluoride?
CH4 + HF →
CH3OH + HF →
CH4 + F2 →
CH3Br + AgF →
Which of the following species can function both as oxidising as well as reducing agent?
Cl-
ClO-
Which of the following is used as an oxidiser in rocket propellants?
Ammonium perchlorate
Alcohol
Acrylic rubber
Polyurethane
