 Multiple Choice Questions
Multiple Choice QuestionsPentavalence in phosphorous is more stable when compared to that of nitrogen even through they belong to same group is due to
reactivity of phosphorous
inert nature of nitrogen
dissimilar electronic configuration
larger size of phosphorous atom
Match List-I with List-II and select the correct answer using the codes given below
| List I (Oxyacid of sulphur) | List II (Oxidation state of sulphur) |
| A. H2SO3 | 1. +1 |
| B. H2S2O6 | 2. +6 |
| C. H2S2O2 | 3. +4 |
| D. H2S2O8 | 4. +5 |
Codes
A B C D
4 3 1 2
A B C D
2 1 3 4
A B C D
4 3 2 1
A B C D
3 4 1 2
Consider the following statements.
1.The aqueous solution of FeCl3 is acidic in nature.
2. Anhyd. FeCl3 can be obtained by heating hydrated ferric chloride.
3. A solution of ferric chloride on standing gives a brown precipitate.
The correct statement (s) is/are
1 and 2
2 and 3
1 and 3
All of these
Mercurous ion (Hg) gives white precipitate on reacting with
Kl solution
H2S gas
K2Cr2O7 solution
HCl solution
In case of ring rest of NO ion, the complex formed has formula [Fe (H2O)5 NO]SO4. The oxidation state of iron in the complex is
+1
+2
+3
+6
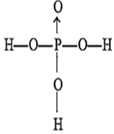
The structure of orthophosphoric acid is
![]()
A.

Ortho phosphoric acid (H3PO4) is a tribasic acid. Hence its structure can be represented as O ← P(OH)3.
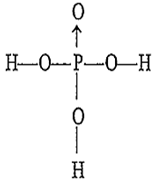
Hence, hybridisation of P in H3PO4 is sp3 and thus, it is tetrahedral in shape.
In the manufacture of ammonia by Haber's process, N2 (g) + 3H2 2NH3(g) + 92.3 KJ which of the following conditions is unfavourable ?
Increasing the temperature
Increasing the pressure
Reducing the temperature
Removing ammonia as it is formed
