 Multiple Choice Questions
Multiple Choice QuestionsWhich one of the following arrangement does not give the correct picture of the trends indicated against it?
F2 > Cl2 > Br2 > I2 : Oxidising power
F2 > Cl2 > Br2 > I2 : Electron gain enthalpy
F2 > Cl2 > Br2 > I2 : Bond dissociation energy
F2 > Cl2 > Br2 > I2 : Bond dissociation energy
Which one of the following ionic species has the greatest proton affinity to form stable compound?
HS-
NH2-
F-
F-
Which one of the following anions is present in the chain structure silicates ?
Si2O76-
(Si2O56-)n
(SiO32-)n
(SiO32-)n
For the following:
(i) I- (ii) Cl- (iii) Br-
the increasing order of nucleophilicity would be:
I- < Br- < Cl-
Cl- < Br- < I-
I- < Cl- < Br-
I- < Cl- < Br-
Which one of the following orders correctly represents the increasing acid strengths of the given acids?
HOCl < HOClO < HOClO2 < HOClO3
HOClO < HOCl < HOClO3 < HOClO2
HOClO2 < HOClO3 < HOClO < HOCl
HOClO2 < HOClO3 < HOClO < HOCl
D.
HOClO2 < HOClO3 < HOClO < HOCl
The acidic character of oxyacids increases with increases in oxidation number. The acidity of oxyacids of chlorine decreases in the following order.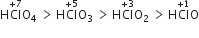
So relative acidic character increases.
The electronegativity difference between N and F is greater than that between N and H yet the dipole moment of NH3 (1.5 D) is larger than that of NF3 (0.2 D). This is because:
In NH3 as well as in NF3 the atomic dipole and bond dipole are in the same direction
In NH3 the atomic dipole and bond dipole are in opposite directions
In NH3 as well as NF3 the atomic dipole and bond dipole are in opposite directions
In NH3 as well as NF3 the atomic dipole and bond dipole are in opposite directions
Which one of the following orders is not in accordance with the property stated against it?
F2 > Cl2 > Br2 > I2 : Oxidising power
HI> HBr> HCl >HF: Acidic property in water
F2 > Cl2 > Br2 > I2: Electrongeativity
F2 > Cl2 > Br2 > I2: Electrongeativity
