 Multiple Choice Questions
Multiple Choice QuestionsCalculate enthalpy for formation of ethylene from the following data
54.1 kJ
44.8 kJ
51.4 kJ
48.4kJ
A system is provided with 50 J of heat and the work done on the system is 10 J. What is the change in internal energy of the system in Joules?
60
40
50
10
Calculate for the reaction,
Na2O (s) + SO3 (g) → Na2SO4 (g) given the following-
(A) Na (s) + H2O (l) → NaOH (s) + H2 (g) ; H° = -146 kJ
(B) Na2SO4 (s) + H2O (l) → 2NaOH (s) + SO3 (g) ; = +418 kJ
(C) 2Na2O (s) + 2H2 (g) → 4Na (s) + 2H2O (l); = +259 kJ
+823 kJ
-581 kJ
-435 kJ
+531 kJ
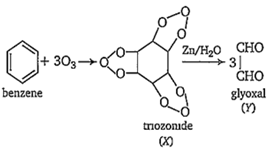
C6H6 + O3 → X Y; X and Y are respectively.
diozonide, glycol
triozonide, glyoxalic acid
triozonide, glyoxal
monoozonide, oxalic acid
C.
triozonide, glyoxal
X and Y are triozonide and glyoxal respectively.
What is the entropy change in JK-1 during the melting of 27.3 g of ice at 0°C? (Latent heat of fusion of ice = 330 Jg-1)
330
12.1
3.3
33
For which one of the following reactions, the entropy change is positive?
H2 (g) + O2 (g) → H2O (l)
Na+ (g) + Cl- (g) → NaCl (s)
NaCl (l) → NaCl (s)
H2O (l) → H2O (g)
Molar heat capacity (Cp) of water at constant pressure is 75 JK-1mol-1. The increase in temperature (in K) of 100 g of water when 1 kJ of heat is supplied to it is
2.4
0.24
1.3
0.13
Calculate for the following cell reaction:
Zn (s) + Ag2O (s) + H2O (l) → Zn (aq) + 2Ag (s) + 2OH- (aq)
E = 0.80 V and E = -0.76 V
-305 kJ/ mol
-301 kJ/ mol
305 kJ/ mol
301 kJ/ mol
The temperature of K at which ΔG = 0, for a given reaction with ΔH =- 20.5 kJ mol-1 and ΔS=-500 JK-1mol-1 is
-410
410
-2.44
2.44
In a reaction, A + B C + D, D, 40% of B has reacted at equilibrium, when 1 mole of A was heated with 1 mole of B in a 10 L closed vessel. The value of Kc is
0.44
0.18
0.22
0.36
