 Multiple Choice Questions
Multiple Choice QuestionsAmong the following, the pair in which the two species are not isostructural, is
SiF4 and SF4
IO and XeO3
BH
PF and SF6
A.
SiF4 and SF4
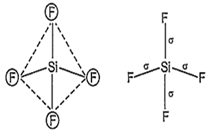
SiF4 and SF4 are not isostructural because SiF4 is tetrahedral due to sp3-hybridisation of Si.
14Si = 1s2, 2s2, 2p6, 3s2 3p2 (In ground state)
14Si = 1s2, 2s2, 2p6, 3s1 3p3 (In excited state)
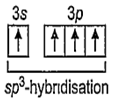
Hence, four equivalent sp3-hybrid orbitals are obtained and they are overlapped by four
p-orbitals of four fluorine atoms on their axes. Thus, it shows following structure:
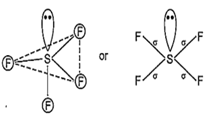
While SF4 is not tetrahedral but it is distorted tetrahedral because in it S is sp3d hybrid and has a lone pair of electron.
16S = 1s2, 2s2 2p6, 3s23p (In ground state)
= 1s2, 2s2 2p6, (In first excitation state)
Hence, sp3d hybrid orbitals are obtained. One orbital is already paired and rest four are overlapped with four p-orbitals of four fluorine atoms on their axes in trigonal bipyramidal form. This structure is distorted from trigonal bi-pyramidal to tetrahedral due to involvement of repulsion between lone pair and bond pair.
The helical structure of protein is stabilized by
dipeptide bond
hydrogen bonds
ether bonds
peptide bonds
The dipole moment of HBr is 1.6 × 10-30 cm and inter-atomic spacing is 1 Å. The % ionic character of HBr is
7
10
15
27
Consider the following ions:
(i) Ni2+ (ii) Co2+ (iii) Cr2+ (iv) Fe3+
(Given, at. no. Cr = 24, Fe = 26, Co = 27, Ni = 28)
The correct order of magnetic moment of these ions is
(i) < (ii) < (iii) < (iv)
(iv) < (ii) < (iii) < (i)
(i) < (iii) < (ii) < (iv)
(iii) < (iv) < (ii) < (i)
