 Multiple Choice Questions
Multiple Choice QuestionsWhich of the following compound possesses the C-H bond with the lowest bond dissociation energy?
Toluene
Benzene
n-pentane
2, 2-dimethyl propane
In the presence of HCl, H2S results the precipitation of group-2 elements but no group-4 elements during qualitative analysis. It is due to
higher concentration of S2-
higher concentration of H+
Lower concentration of S2-
Lower concentration of H+
Which of the following is most likely to show optical isomerism ?
HC≡C--C≡CH
HC≡C--CH3
HC≡C--H
HC≡C-C (Cl) = CH2
Acetylene reacts with HCN in the presence of Ba(CN)2 to yield :
1, 1-dicyanoethane
1, 2-dicyanoethane
vinyl cyanide
None of the above
C.
vinyl cyanide
Acetylene reacts with HCN in the presence of Ba(CN)2 to yield vinyl chloride.
The following compound will undergo electrophilic substitution more readily than benzene:
nitrobenzene
benzoic acid
benzaldehyde
phenol
The correct statement about the compounds (a),(b)and (c) is :
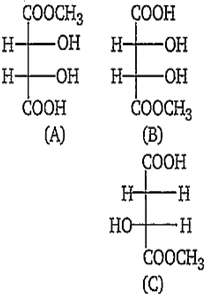
(A) and (B) are identical
(A) and (B) are diasteromers
(A) and (B) are not enantiomers
(A) and (B) are enantiomers
