 Multiple Choice Questions
Multiple Choice QuestionsWhich of the following will have a meso-isomer also?
2-chlorobutane
2,3-dichlorobutane
2,3-dichloropentane
2-hydroxypropanoic acid
B.
2,3-dichlorobutane
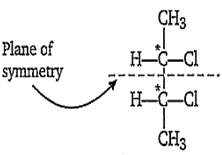
Among the given options, 2, 3-dichlorobutane have a meso-isomer.
In this reaction
CH3CHO + HCN → CH3CH(OH)CN CH3CH(OH)COOH
an asymmetric centre is generated. The acid obtained would be
50% D + 50% L-isomer
20% D + 80% L-isomer
D-isomer
L-isomer
According to IUPAC nomenclature sodium nitroprusside is named as
sodium pentacyanonitrosyl ferrate (II)
sodium pentacyanonitrosyl ferrate (III)
sodium nitroferricyanide
sodium nitroferrocyanide
CH3CH2OH and CH3OCH3 are the examples of
chain isomerism
functional isomerism
position isomerism
metamerism
In the following reaction
RCH2COOH Y
The major maounts of X and Y are
RCHBrCONH2; RCH(NH2)COOH
RCHBrCOOH; RCH(NH2)COOH
RCH2COBr; RCH2COONH4
RCHBrCOOH; RCH2CONH2
The monosaccharides having anomeric carbon atom are
geometrical isomers
α- and β- optical isomers
having symmetrical carbon atoms
None of the above
In Kjeldahl's method of estimation of nitrogen, CuSO4 acts as
oxidising agent
reducing agent
catalytic agent
hydrolysis agent
