 Multiple Choice Questions
Multiple Choice QuestionsA metal 'M' reacts with N2 to give a compound 'A' (M3N). 'A' on heating at high temperature gives back 'M' and 'A' on reacting with H2O give a gas B. 'B' turns CuSO4 solution blue on passing through it. M and B can be
Al and NH3
Li and NH3
Na and NH3
Mg and NH3
CH3CH2CH(OH)CH3 and CH3(CH2)2CH2OH can be distinguished by
Lucas test
Iodoform test
Victor-Meyer's test
All of the above
The compound which does not exhibit geometrical isomerism, is
CH3CH=CHCOOH
Br-CH=CH-Br
C6H5CH=NOH
![]()
Which of the following is the most reactlve towards ring nitration ?
benzene
toulene
m-xylene
mesitylene
D.
mesitylene
Nitration of benzene is an electrophlic substitution reaction and presence of electron releasing group (like -CH3, -C2H5) activates the ring towards electrophilic substitution at ortho/para position. Mesitylene have three CH3 groups.
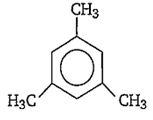
Thus, it is most reactive towards ring nitration.
Racemic compound has
equimolar mixture of enantiomers
1 : 1 mixture of enantiomer and diastereomer
1 : 1 mixture of diastereomers
1 : 2 mixture of enantiomers
In which of the following ways does the hydride ion tend to function ?
An electrophile
A nucleophile
A free radical
An acid
