 Multiple Choice Questions
Multiple Choice QuestionsGenerally, the first ionization energy increases along a period. But there are some exceptions. One which is not an exception is
N and O
Na and Mg
Mg and Al
Be and B
For alkali metals, which one of the following trends is incorrect?
Hydration energy : Li > Na > K > Rb
Ionization energy : Li > Na > K > Rb
Density : Li < Na < K < Rb
Atomic size : Li < Na < K < Rb
Which one of the following is true?
NaOH is used in the concentration of bauxite ore
NaOH is a primary standard in volumetric analysis
Manganous hydroxide is soluble in excess of NaOH solution
NaOH solution does not react with Cl
The reaction between sodium and water can be made less vigorous by
adding a little alcohol
amalgamating sodium
adding a little acetic acid
lowering the temperature
Alkali metals have negative reduction potential and hence they behave as
oxidising agents
Lewis bases
reducing agents
electrolytes
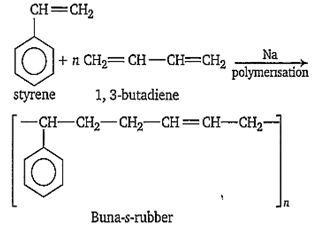
The s-block element used as a catalyst in the manufacture of Buna-S rubber is
Ca
Ba
Na
Mg
C.
Na
Butadiene (75%) and styrene (25%) polymerise in the presence of sodium to give Buna-S-rubber.
When limestone is heated, CO2 is given off. The metallurgical operation is
smelting
reduction
calcination
roasting
On heating potassium permanganate, one of the following compound is not obtained.
O2
MnO
MnO2
K2MnO4
