Multiple Choice Questions
Multiple Choice QuestionsWhich one of the following statements is correct?
Hybrid orbitals do not form σ bonds.
Lateral overlap of p-orbitals or p- and d-orbitals produces -bonds.
The strength of bonds follows the order-
σp-p < σs-s < p-p
s-orbitals do not form σ bonds.
Which of the following is a proper match?
| Shape | |
| A. 0.115 - 0.225 | i. Triangular |
| B. 0.225 - 0.414 | ii. Tetrahedral |
| C. 0.414 - 0.732 | iii. Cubic |
| D. 0.732 - 1 | iv. Octahedral |
A - i; B - ii; C - iv; D - iii
A - iii; B - ii; C - iv; D - i
A - i; B - iii; C - ii; D - iv
A - ii; B - iv; C - i; D - iii
The correct order of increasing C-O bond length of CO, CO2 and CO is
CO < CO2 < CO
CO2 < CO < CO
CO < CO < CO2
CO < CO2 < CO
Peroxide ion ......
(i) has five completely filled antibonding molecular orbitals
(ii) is diamagnetic
(iii) has bond order one
(iv) is isoelectronic with neon
Which one of these is correct?
(ii) and (iii)
(i), (ii) and (iv)
(i), (ii) and (iii)
(i) and (iv)
C-H bond energy is about 101 kcal/ mol for methane, ethane and other alkanes but is only 77 kcal/ mol for C-H bond of CH3 in toluene. This is because :
of inductive effect due to -CH3 in toluene
of the presence of benzene ring in toluene
of resonance among the structures of benzyl radical in toluene
aromaticity of toluene
Which one of the following does not have sp2 hybridised carbon ?
Acetone
Acetic acid
Acetonitrile
Acetamide
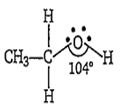
The C-O-H bond angle in ethanol is nearly
90°
104°
120°
180°
A.
90°
In ethanol the oxygen of -OH group is bonded to the sp3 hybridised carbon by a sigma bond. The -C-O-H bond angle in ethanol is less than the tetrahedral angle (109° 28) due to larger repulsions between the lone pairs of repulsions between the lone pairs of oxygen. Hence it is 104° in ethanol.