 Multiple Choice Questions
Multiple Choice Questions1 mole of methyl amine on reaction with nitrous acid gives at NTP
1.0 L of nitrogen
22.4 L of nitrogen
11.2 L of nitrogen
5.6 L of nitrogen
The major product P in the following reaction is
CH3-CH=CH2 P
CH3CH2CH2I
CH3-CH(I)-CH3
CH2(I)-CH=CH2
CH2(I)-CH2(I)
Which of the following will exhibit cis-trans isomerism?
CH2Br-CH2Br
CBr3 - CH3
CHBr = CHBr
CBr2= CH2
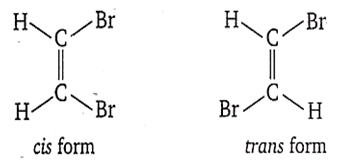
C.
CHBr = CHBr
Alkene shows geometrical isomerism due to restricted rotation of double bond. It is because the position of atoms or groups attached to the carbon atoms of the double bond gets fixed. If same group or atom is attached with double bond, then alkene does not show geometrical isomerism.
The IUPAC name of CH3-CH=CH-C≡CH is
pent-3-en-1-yne
pent-3-en-4-yne
pent-2-en-4-yne
pent-2-en-3-yne
The correct IUPAC name of ![]() is
is
1-cyclopropyl cyclobutane
1, 1-dicyclobutane
1-cyclobutane- 1- cyclopropane
none of the above
Geometrical isomerism is possible in
acetone- oxime
isobutene
acetophenone-oxime
benzophenone-oxime
IUPAC name of the following compound is:
CH3CH2C(Br) = CH - Cl
2-bromo-1-chloro butene
1-chloro-2-bromo butene
3-chloro-2-bromo butene-2
none of the above
Quantitative measurement of nitrogen in an organic compound is done by the method :
Berthelot method
Belstein method
Lassaigne test
Kjheldahl method
Brown ring in the test of NO is formed due to the formation of :
FeSO4.NO
[Fe(SO4)2.NO]H2O
Fe2(SO4)3.NO
None of these
Which kind of fission is favoured by sunlight ?
Heterolytic fission
Homolytic fission
Both (a) and (b)
None of these
