CBSE Multiple Choice Questions
Multiple Choice QuestionsTwo different isotherms representing the relationship between pressure p and volume V at a given temperature of the same ideal gas are shown for masses m1 and m2 , then
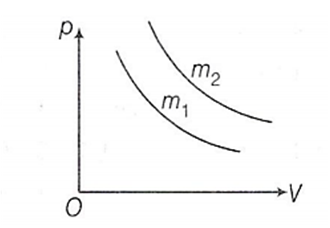
Nothing can be predicted
m1 < m2
m1 = m2
m1 > m2
The temperature of the cold junction of thermocouple is 0°C and the temperature of hot junction is To C. The emf is E = 16T - 0.04T2 µV. The inversion temperature Tf is
300o C
200o C
500o C
400o C
Degree of freedom for polyatomic gas
≥ 4
≥5
≥6
≥7
Two rigid boxes containing different ideal gases are placed on table. Box A contains one mole of nitrogen at temperature T0 , while box B contains 1 mole of helium at temperature 7/3 T0. The boxes are then put into thermal contact with each other and heat flows between them until the gases reach a common final temperature (ignore the heat capacity of boxes) then the final temperature of gases, Tf in terms of T0 is
The temperature of a gas is raised from 27° C to 927° C. The root mean square speed
gets halved
gets doubled
is times the earlier value
remains the same
Assertion: Vibrational energy of diatomic molecule corresponding to each degree of freedom is kB T.
Reason: For every molecule, vibrational degree of freedom is 2.
If both assertion and reason are true and reason is the correct explanation of assertion.
If both assertion and reason are true but reason is not correct explanation of assertion.
If assertion is true but reason is false.
If both assertion and reason are false.
For a gas = 0.67. This gas is made up of molecules which are
monoatomic
polyatomic
mixture of diatomic and polyatomic molecules
diatomic
Pressure of an ideal gas is increased by keeping temperature constant. What is effect on kinetic energy of molecules?
Increase
Decrease
No change
Cannot be determined
1 mole of H2 gas is contained in a box of volume V = 1.00 m3 at T = 300 K. The gas is heated to a temperature of T = 3000 K and the gas gets converted to a gas of hydrogen atoms. The final pressure would be (considering all gases to be ideal)
same as the pressure initially
2 times the pressure initially
10 times the pressure initially
20 times the pressure initially
A horizontal tube of length l closed at both ends, contains an ideal gas of molecular weight M. The tube is rotated at a constant angular velocity ω about a vertical axis passing through an end. Assuming the temperature to be uniform and constant. If p1 and p2 denote the pressure at free and the fixed end respectively, then choose the correct relation

