CBSE Multiple Choice Questions
Multiple Choice QuestionsPressure versus temperature graph of an ideal gas is as shown in figure. Density of the gas at point A is ρ0 Density at point B will be
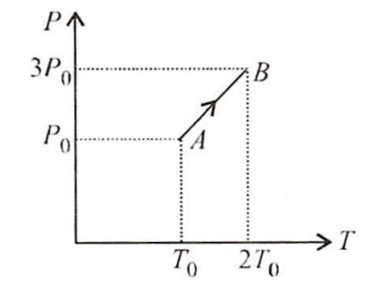
ρ0
ρ0
ρ0
2ρ0
If at the same temperature and pressure, the densities of two diatomic gases are d1 and d, respectively, the ratio of mean kinetic energy per molecule of gases will be
1 : 1
:
vrms , vav and vmp are root mean square, average and most probable speeds of molecules of a gas obeying Maxwellian velocity distribution. Which of the following statements is correct?
vrms < vav < vmp
vrms > vav > vmp
vmp > vrms > vav
vmp > vrms > vav
A monoatomic gas supplied the heat Q very slowly keeping the pressure constant. The work done by the gas will be:
The value of the gas constant (R) calculated from the perfect gas equation is 8.32 J/g mol-K, whereas its, value calculated from the knowledge of CP and Cv of the gas is 1.98 cal/g mol-K. From this data value of J is
4.16 J/cal
4.18 J/cal
4.20 J/cal
4.22 J/cal
The velocities of sound at the same temperature in two monoatomic gases of densities ρ1 and ρ2 and ν1 and ν2 respectively. If ρ1 / ρ2 = 4, then the value of ν1/ ν2 is
1/4
2
1/2
4
The average velocity of the molecules in a gas in equilibrium is
proportional to
proportional to T
proportional to T2
equal to zero
Assertion: The root mean square and most probable speeds of the molecules in a gas are the same.
Reason: The Maxwell distribution for the speed of molecules in a gas is symmetrical.
If both assertion and reason are true and reason is the correct explanation of the assertion
If both assertion and reason are true but reason is not the correct explanation of the assertion
If assertion is true, but reason is false
Both assertion and reason are false statements
Two balloons are filled, on with pure He gas and other by air, respectively. If the pressure and temperature of these balloons arc same then the number of molecules per unit volume is
more in the He filled balloon
same in both balloons
more in air filled balloon
in the ratio of 1 : 4
When the temperature of an ideal gas is increased from 27°C to 227°C, its rms speed is changed from 400 metre/sec to vs. The vs is :
516 m/s
450 m/s
310 m/s
746 m/s

