CBSE Multiple Choice Questions
Multiple Choice QuestionsTwo rods A and B of different materials are welded together as shown in the figure. Their thermal conductivities are K1 and K2. The thermal conductivity of the composite rod will be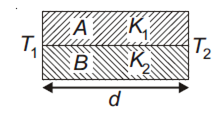
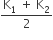
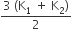
k1 + k2
k1 + k2
A monatomic gas at pressure p1 and V1 is compressed adiabatically to 1/8 th its original volume. What is the final pressure of the gas?
64 p1
p1
16 p1
16 p1
If ΔU and ΔW represent the increase in internal energy and work done by the system respectively in the thermodynamical process, which of the following is true?
ΔU = - ΔW, in a adiabatic process
ΔU = ΔW, in an isothermal process
ΔU = ΔW, in an adiabatic process
ΔU = ΔW, in an adiabatic process
The molar specific heat at constant pressure of an ideal gas is (7/2)R. The ratio of specific heat at constant pressure to that at constant volume is
7/5
8/7
5/7
5/7
The internal energy change in a system that has absorbed 2 Kcal of heat and done 500 J of work is
8900 J
6400 J
5400 J
5400 J
In producing chlorine through electrolysis 100 W power at 125 V is being consumed. How much chlorine per min is liberated? ECE of chlorine is 0.367 x 10-6 kg/C
17.6 mg
21.3 mg
24.3 mg
24.3 mg
A Carnot engine whose sink is at 300 K has an efficiency of 40%. By how much should the temperature of source be increased so as to increase its efficiency by 50% of original efficiency?
275 K
175 K
250 K
250 K
If Q, E andW denote respectively the heat added, change in internal energy and the work done in a closed cycle process, then
W =0
Q = W = 0
E=0
E=0
Thermodynamic processes are indicated in the following diagram.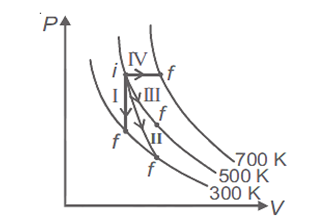
Match the following
| Column-1 | Column-2 |
| P. Process I | a. Adiabatic |
| Q. Process II | b. Isobaric |
| R. Process III | c. Isochoric |
| S. Process IV | d. Isothermal |
P → a, Q → c, R → d, S → b
P → c, Q → a, R → d, S → b
P → c, Q → d, R → b, S → a
P → c, Q → d, R → b, S → a
In a thermodynamic process which of the following statements is not true?
In an adiabatic process, the system is insulated from the surroundings
In an isochoric process pressure remains constant
In an isothermal process, the temperature remains constant
In an isothermal process, the temperature remains constant

