 Short Answer Type
Short Answer TypeAlthough phenoxide ion has more number of resonating structures than Carboxylate ion, Carboxylic acid is a stronger acid than phenol. Give two reasons.
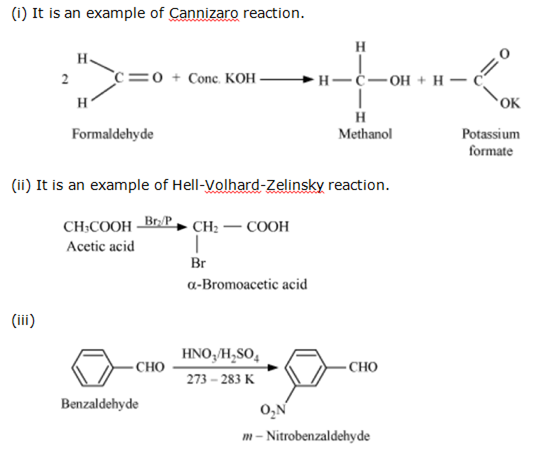
Complete the following reactions:

 Long Answer Type
Long Answer TypeRate constant ‘k’ of a reaction varies with temperature ‘T’ according to the equation:
![]()
where Ea is the activation energy.
When a graph is plotted for ![]() a straight line with a slope of -4250 K is obtained. Calculate ‘Ea’ for the reaction.
a straight line with a slope of -4250 K is obtained. Calculate ‘Ea’ for the reaction.
(R = 8.314 JK-1 mol-1)
How will you bring about the following conversions?
(i) Propanone to propane
(ii) Benzoyl chloride to benzaldehyde
(iii) Ethanal to but-2-enal
Give simple chemical tests to distinguish between the following pairs of compounds:
(i) Ethanal and Propanal
(ii) Benzoic acid and Phenol
