 Short Answer Type
Short Answer Type(i) What is the role of t-butyl peroxide in the polymerization of ethene?
(ii) Identify the monomers in the following polymer:
OR
Write the mechanism of free radical polymerization of ethene.
(i) In polymerisation of ethene, the source of free radical is needed to initiate the chain reaction. Such free radicals are usually produced by the decomposition of peroxides like t- butyl peroxide or benzoyl peroxide.
(ii)The given polymer is nylon 6, 6.
Monomers of nylon 6,6 are adipic acid (HOOC(CH2)4COOH)and hexamethylenediamine (H2N(CH2)6NH2).
(iii)Elastomers of rubbers have the weakest intermolecular forces of attraction, while fibres have the strongest intermolecular forces of attraction. Plastics have intermediate forces of attraction.
The increasing order of intermolecular forces of attraction of the given polymers is as follows:
Terylene> Polystyrene > Buna-S
Or
The mechanism of free radical polymerization of ethene.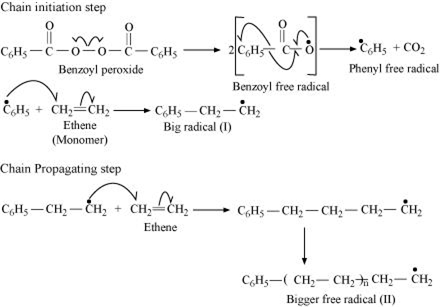
Due to hectic and busy schedule, Mr. Angad made his life full of tensions and anxiety. He started taking sleeping pills to overcome the depression without consulting the doctor. Mr. Deepak, a close friend of Mr. Angad, advised him to stop taking sleeping pills and suggested to change his lifestyle by doing Yoga, mediation and some physical exercise. Mr. Angad followed his friend's advice and after few days he started feeling better.
After reading the above passage, Ans the following:
(i)What are the values (at least two) displayed by Mr. Deepak?
(ii)Why is it not advisable to take sleeping pills without consulting a doctor?
(iii)What are tranquillizers? Give two examples.
 Long Answer Type
Long Answer Typea) Calculate the freezing point of the solution when 1.9 g of MgCl2 (M = 95 g mol−1) was dissolved in 50 g of water, assuming MgCl2 undergoes complete ionization.
(Kf for water = 1.86 K kg mol−1)
(b)
(i) Out of 1 M glucose and 2 M glucose, which one has a higher boiling point and why?
(ii) What happens when the external pressure applied becomes more than the osmotic pressure of solution?
OR
(a)When 2.56 g of sulphur was dissolved in 100 g of CS2, the freezing point lowered by 0.383 K. Calculate the formula of sulphur (Sx).
(Kf for CS2 = 3.83 K kg mol−1, Atomic mass of sulphur = 32 g mol−1]
(b)Blood cells are isotonic with 0.9% sodium chloride solution. What happens if we place blood cells in a solution containing
(i)1.2% sodium chloride solution?
(ii)0.4% sodium chloride solution?
(a) Account for the following:
(i)Ozone is thermodynamically unstable.
(ii)Solid PCl5 is ionic in nature.
(iii)Fluorine forms only one oxoacid HOF.
(b) Draw the structure of
(i) BrF5
(ii) XeF4
OR
(i)Compare the oxidizing action of F2 and Cl2 by considering parameters such as bond dissociation enthalpy, electron gain enthalpy and hydration enthalpy.
(ii)Write the conditions to maximize the yield of H2SO4 by contact process.
(iii)Arrange the following in the increasing order of property mentioned:
(a)H3PO3, H3PO4, H3PO2 (Reducing character)
(b)NH3, PH3, AsH3, SbH3, BiH3 (Base strength)
Write the structures of A, B, C, D and E in the following reactions:
Or
(a)Write the chemical equation for the reaction involved in Cannizzaro reaction.
(b)Draw the structure of the semicarbazone of ethanal.
(c)Why pKa of F-CH2-COOH is lower than that of Cl−CH2−COOH?
(d)Write the product in the following reaction: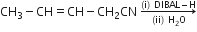
(e)How can you distinguish between propanal and propanone?
