 Short Answer Type
Short Answer TypeWhen concentrated sulphuric acid was added to an unknown salt present in a test tube a brown gas (A) was involved. This gas intensified when copper turnings were added to this test tube. On cooling, the gas (A) changed into a colourless solid (B).
(i) Identify (A) and (B)
(ii) Write the structures of (A) and (B)
(iii) Why does gas (A) change to solid on cooling
(i) A is NO2 gas (because NO3- salt reacts with conc. H2SO4 to give NO2 gas which is brown). (B) is N2O4
(ii)
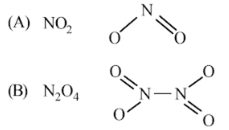
(iii) NO2 (A) is an odd electron-molecule. Thus, to become stable, it decreases to give N2O4 which is a colourless solid.
Out of chlorobenzene and benzyl chloride, which one gets easily hydrolysed by aqueous NaOH and why?
