 Short Answer Type
Short Answer TypeSolution A is a strong.
Solution B is a weak acid
Solution C is a strong alkali.
Which solution will give a gelatinous white precipitate with zinc sulphate solution? The precipitate disappears when an excess of the solution is added.
Give one chemical test to distinguish between the following pairs of compounds:
Zinc sulphate solution and zinc chloride solution.
Give one chemical test to distinguish between the following pairs of compounds:
Iron (II) chloride solution and iron (III) chloride solution.
Give one chemical test to distinguish between the following pairs of compounds:
Calcium nitrate solution and calcium chloride solution.
g) (i) Calcium carbide is used for the artificial ripening of fruits. Actually, the fruit ripens because of the heat evolved while calcium carbide reacts with moisture. During this reaction calcium, hydroxide and acetylene gas are formed. If 200 Cm3of acetylene is formed from a certain mass of calcium carbide, find the volume of oxygen required and carbon dioxide formed during the complete combustion. The combustion reaction can be represented as below:
2C2H2(g) +5O2(g) ----> 4CO2(g) +2H2O2(g)
Correct the following statement:
For example: Chlorine is a bleaching agent.
Should read : moist chlorine is a bleaching agent.
Equal masses of all gases under identical condition contain the same number of molecules.
A gas cylinder contains 24 x1024 molecules of nitrogen gas. If Avogadro’s number is 6 x1023 and the relative atomic mass of nitrogen is 14 calculate:
i) Mass of nitrogen gas in the cylinder
ii) Volume of nitrogen at STP in dm3
i) 6 x 1023 molecules of nitrogen weigh =2 x 14 g.
therefore,
24 x 1024 molecules of nitrogen weigh =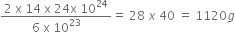
ii) 6 x 1023 molecules of N2 occupy 22.4 l at STP
24 x 1024 molecules of N2 occupy =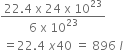
Commercial sodium hydroxide weighing 30 g has some sodium chloride in it. The mixture on dissolving in water and subsequent treatment with excess silver nitrate solution formed a precipitate weighing 14.3g. what is the percentage of sodium chloride in the commercial sample of sodium hydroxide? The equation for the reaction is
NaCl +AgNO3 ---> AgCl +NaNO3
[relative molecular mass of NaCl =58; AgCl =143]
A certain gas ‘X’ occupies a volume of 100 cm3 S.T.P and weigh 0.5 g find its relative molecular mass .
