 Short Answer Type
Short Answer Type(c) complete the following calculation. Show working for complete credit:
I) calculate the mass of calcium that will contain the same number of atom as are present in 3.2 gm of sulphur.
[atomic masses : S= 32, Ca= 40]
complete the following calculation. Show working for complete credit:
if 6 liters of hydrogen and 4 liters of chlorine are mixed and exploded and if water is added to the gases formed, find the volume of the residual.
Complete the following calculation. Show working for complete credit:
If the empirical formula of a compound is CH and it has a vapour density of 13 find the molecular formula of the compound.
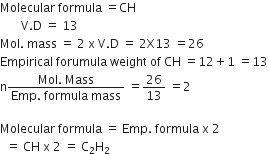
Q5. (a) consider the following reaction and based on the reaction answer the questions that follows:”
NH4)Cr2O7 (heat) -->N2(g) +4H2O (g) +Cr2O3
Calculate:
i) The quantity in moles of NH4)Cr2O7 if 63 gm of NH4)Cr2O7 is heated.
ii) The quantity in moles of nitrogen formed.
iii) The volume in litres of dm3 of N2 evolved at S.T.P
iv) The mass in grams of Cr2O3 formed at the same time
[ atomic masses : H=1, Cr=52, N=14]
State one relevant observation for the following:
At the anode when aqueous copper sulphate solution is electrolyzed using copper electrodes.
Nothing is left at the anode as copper atoms form copper ions and migrate towards the cathode.
Give appropriate scientific reason for the following statement:
During electrolysis of molten lead bromide, graphite anode is preferred to other electrodes.
The electrical conductivity of acetic acid is less in comparison to the electrical conductivity of dilute sulphuric acid at a given concentration ?
Differentiate between the terms strong electrolyte and weak electrolyte.
(stating and two differences)
i) Copy and complete the following table:
|
|
Anode |
Electrolyte |
|
Purification |
|
|
ii) Write the equation taking place at the anode:
