 Short Answer Type
Short Answer TypeA gas cylinder contains 12 x 1024 molecules of oxygen gas.
if Avogadro's number is 6 x 1023; Calculate:
i) The mass of oxygen present in the cylinder
ii) The volume of oxygen at S.T.P present in the cylinder. [O=16]
A gaseous hydrocarbon coantains 82.76% of carbon. Given that its vapour density is 29, find its molecular formula. [C=12, H=1]
The equation 4NH3 +5O2 ---> 4NO +6H2O, represents the catalytic oxidation of ammonia. If 100cm3 of ammonia is used. Calculate the volume of oxygen required to oxidise the ammonia completely.
By drawing an electron dot diagram show the formation of Ammonium ion.
[Atomic No.: N =7 and H = 1]
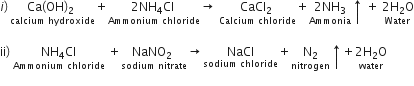
Name the gas evolved when the following mixtures are heated:
(i) Calcium hydroxide and Ammonium Chloride
(ii) Sodium Nitrite and Ammonium Chloride.
Write balanced chemical equations for each of the following:
i) When excess of ammonia is treated with chlorine
ii) An equation to illustrate the reducing nature of ammonia.
A,B,C and D summarise the properties of sulphuric acid depending on whether it is dilute or concentrated.
A= Typical acid property
B= Non-volatile acid
C= Oxidizing agent
D= Dehydrating agent
Choose the property (A, B, C or D) depending on which relevant to each of the following:
i) Preparation of hydrogen chloride gas.
ii) Preparation of Copper sulphate from copper oxide.
iii) The action of conc. Sulphuric acid on sulphur.
Give a reason why:
i) Sodium chloride will conduct electricity only in fused or aqueous solution state.
ii) in the electroplating of an article with silver, the electrolyte sodium argento-cyanide solution is preferred over silver nitrate solution.
iii) Although copper is a good conductor of electricity, it is a non-electrolyte.
i) Name the solution used to react with Bauxite as a first step in obtaining pure aluminium oxide, in the Bayer's process.
ii) Write the equation for the reaction where the aluminium oxide for the electrolytic extraction of aluminium is obtained by heating aluminium hydroxide.
iii) Name the compound added to pure alumina to lower the fusion temperature during the electrolytic reduction of alumina
iv) Write the equation for the reaction that occurs at the cathode during the extraction of aluminium by electrolysis.
v) Explain why it is preferable to use a number of graphite electrodes as anode instead of a single electrode, during the above electrolysis.
State what would you observe when:
i) Washing soda Crystal are exposed to the atmosphere.
ii) The salt ferric chloride is exposed to the atmosphere.
