 Short Answer Type
Short Answer TypeWrite balanced equations for each of the following reactions : Â Â
(i) Dilute hydrochloric acid is added to sodium thiosulphate solution.
(ii) Sodium hydroxide solution is added to silver nitrate solution
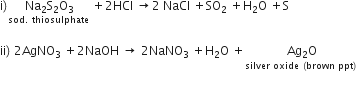
 Explain the following with at least one example :  Â
(i) Keto-enol tautomerism.
(ii) Carbylamine reaction.
(iii) Rosenmund’s reduction.
(iv) Haloform reaction.
 Give one reason each for the following : Â
(i) Acetone reacts with hydroxylamine to form only one product which has no geometrical isomer, but acetaldehyde reacts with hydroxylamine to form a product which has two geometrical isomers.
(ii) Direct nitration of aniline is not possible.
Write balanced equations for the preparation of: Â
(i) DDT
(ii) Urotropine
(iii) Biuret
Give balanced equations for the reactions given below: Â Â
(i) Urea is warmed with a dilute solution of sodium hydroxide.
(ii) Methyl isocyanide is warmed with dilute hydrochloric acid.
(iii) Sucrose is warmed with concentrated nitric acid.
(iv) Aniline is treated with a mixture of NaNO2Â and excess ofHCl at low temperature.
 How would you convert the following :
(i) Methyl amine to ethyl amine
(ii) Benzoic acid to benzene
(iii) Glucose to fructose
Give a chemical test to distinguish between :Â
(i) Urea and oxalic acid
(iiAcetaldehyde and acetone.
i) What is a specific rotation ? Â Â
(ii) 1. What is optical activity ?
2. Name the apparatus used to measure optical activity.
3. State the two necessary conditions for a compound to show optical activity. Give one example of a compound which shows optical activity.

