 Short Answer Type
Short Answer TypeA compound AB has a simple cubic structure and has molecular mass 99. Its density is 3.4 g cm-3. What will be the edge length of the unit cell?
The molecular weight of an organic compound is 58 g mol-1. What will be the boiling point of a solution containing 48 grams of the solute in 1200 grams of water?
[Kb for water = 0.213 C kg mole-1; Boiling point of water = 1000 C]
In the first order reaction,10% of the reactant is consumed in 25 minutes. Calculate:
i) The half-life period of the reaction.
ii) The time required for completing 87.5% of the reaction.
Consider the following cell reaction at 298K:
2Ag+ +Cd--> 2Ag +Cd2+
The standard reduction potentials (E0) for Ag+/Ag and Cd2+/Cd are 0.80V and -0.40 V respectively:
1) write the cell representation.
2) what will be the emf of the cell if the concentration of Cd2+ os 0.1 M and that of Ag+ is 0.2M?
3) Will the cell work spontaneously for the condition given in (2) above?
From the given chemical equation:
2Ag+ +Cd--> 2Ag +Cd2+
Ag+/Ag = 0.80V
Cd2+/Cd =-0.40V
The Ered for silver> Ered for cadmium, sliver ion will be reduced and cadmium metal will be oxidized.
anode reaction: Cd--> Cd2+ +2e-
cathode reaction: 2Ag+ +2e- --> 2Ag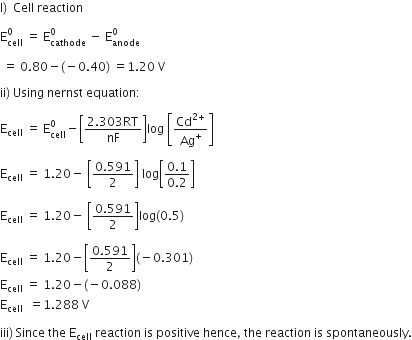
What is a buffer solution? How is it prepared to explain the buffer action of as basic buffer with a suitable example?
Explain the following:
i) when NaCl is added to AgNO3 solution, a white precipitate is formed.
ii) An aqueous solution of ammonium chloride is acidic in nature.
A 0.05 M NH4OH solution offers the resistance of 50 ohms to a conductivity cell at 298K. If the cell constant is 0.50 cm-1 and molar conductance of NH4OH at infinite dilution is 471.4 ohm-1cm2mol-1, calculate:
i) Specific conductance
ii) Molar conductance
iii) Degree of dissociation
Water acts as Bronsted acid as well as a Bronsted base. Give one example each to illustrate this statement.
What type of isomerism is exhibited by the following pairs of compounds:
i) [PtCl2(NH3)4]Br2 and [PtBr2(NH3)4Cl2
ii) [Cr(SCN)(H2O)5]2+ and [Cr(NCS)(H2O)5]2+
Give balanced equations for the following reaction:
i) Silver nitrate si added to the dilute solution of sodium thiosulphate.
ii) Potassium dichromate is treated with acidified ferrous sulphate solution.
iii) Phosphorus reacts with conc. sulphuric acid.
