 Short Answer Type
Short Answer TypeHow will you obtain pure potassium permanganate (KMnO4) crystals from its ore, pyrolusite? Give the step involved and the reactions.
For the reaction: 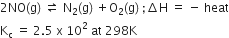
what will happen to concentration of N2 if:
i) Temperature is decreased to 273K
ii) Pressure is reduced.
i) According to Le Châtelier's principle, an decrease in temperature would shift the equilibrium to right and-and value of Kc increased.
ii) increase or decrease in pressure affect equilibrium only when the number of moles varies. Since the number of moles on both side reactant and product is same hence there will be not any change.
What will be the value of van't Hoff factor (i) Of benzoic acid if it dimerised in aqueous solution? How will the experimental weight vary as compared to the normal molecular weight?
