 Multiple Choice Questions
Multiple Choice QuestionsThe overlapping of orbitals in benzene is of the type
sp-sp
p-p
sp2-sp2
sp3-sp3
C.
sp2-sp2
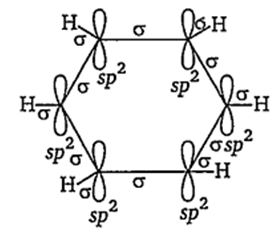
The molecular orbital picture of benzene shows that in it all the six carbon atoms are sp2 hybridised. Out of these three sp2 hybrid orbitals of each C atom, two orbitals overlap with sp2 hybrid orbitals of adjacent C atoms to form six C—C single bonds. The remaining sporbital of each C atom overlaps with s-orbital of each hydrogen atom to form six C—H single sigma bonds. Each C atom is now left with one unhybridised p-orbital perpendicular to the plane of the ring.
One mole of which of the following has the highest entropy?
Liquid nitrogen
Hydrogen gas
Mercury
Diamond
A gas deviates from ideal behaviour at a high pressure because its molecules
attract one another
show the Tyndall effect
have kinetic energy
are bound by covalent bonds
If one mole of ammonia and one mole of hydrogen chloride are mixed in a closed container to form ammonium chloride gas, then
ΔH > ΔU
ΔH = ΔU
ΔH < ΔU
there is no relationship
Three moles of PCl3 three moles of PCl3, and two moles of Cl, are taken in a closed vessel. If at equilibrium the vessel has 1.5 moles of PCl5 the number of moles of PCl3, present in it is
5
3
6
4.5
An octahedral complex is formed when hybrid orbitals of the following type are involved
sp3
dsp2
d2sp3
sp2d2
