 Multiple Choice Questions
Multiple Choice QuestionsA buffer solution is prepared by mixing 0.1 M ammonia and 1.0 M ammonium chloride. At 298 K, the pKb of NH4OH is 5.0. The pH of the buffer is
10.0
9.0
6.0
8.0
Among NH3, HNO3, NaN3 and Mg3N2 the number of molecules having nitrogen in negative oxidation state is
1
2
3
4
Born-Haber cycle may be used to calculate
electronegativity
mass number
oxidation number
electron affinity
The heat of formation for CO2 (g), H2O (l) and CH4 (g) are -400 kJ mol-1, -280 kJ mol-1 and -70 kJ mol-1 respectively. The heat of combustion of CH4 in kJ mol-1 is
890
-160
-890
-90
The molecule having zero dipole moment is
CH2Cl2
BF3
NF3
ClF3
B.
BF3
The dipole moment of BF3 molecule is zero due to its symmetrical (triangular planar) structure. The three fluorine atoms lie at the corners of an equilateral triangle with boron at the centre. Thus, the vectorial addition of the dipole moments of the three bonds gives a netsum of zero.
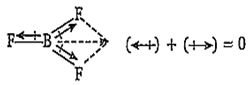
Oxygen and sulphur both are the member of same group in periodic table but H2O is liquid while H2S is gas because
molecular weight of water is more
electronegativity of sulphur is more
H2S is weak acid
water molecules are having weak hydrogen bonds between them
