 Multiple Choice Questions
Multiple Choice QuestionsThe pKa values of four carboxylic acids are given below. Identify the weakest carboxylic acid.
4.89
1.28
4.76
2.56
Which one of the following is an example of disproportionation reaction?
3Cl2 (g) + 6OH- (aq) → ClO (aq) + 5Cl- (aq) + 3H2O (l)
Ag2+ (aq) + Ag (s) → 2Ag+ (aq)
Zn (s) + CuSO4 (aq) → Cu (s) + ZnSO4 (aq)
2KClO3 (s) → 2KCl (s) + 3O2 (g)
Observe the following statements-
i. Heavy water is harmful for the growth of animals.
ii. Haevy water reacts with Al4C3 and forms deyterated acetylene.
iii. BaCl2.2D2O is an example of interstitial deuterate.
The correct statements are-
1 and 3
1 and 2
1, 2 and 3
2 and 3
How many corners of SiO4 units are shared in the formation of three dimensional silicates?
3
2
4
1
Na2S2O3 reacts with moist Cl2 to form Na2SO4, HCl and X. Which one of the following is X?
H2S
SO2
SO3
S
Cataract and skin cancer are caused by
depletion of nitric oxide
depletion of ozone layer
increase in methane
depletion of nitrous oxide
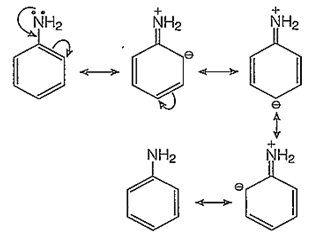
Assertion (A) : NH2 group of aniline is ortho, para directing in electrophilic substitutions.
Reason (R) : -NH2 group stabilises the arenium ion formed by the ortho, para attack of the electrophile.
The correct answer is
Both (A) and (R) are correct, (R) is the correct explanation of (A).
Both (A) and (R) are correct, (R) is not the correct explanation of (A).
(A) is correct, but (R) is not correct.
(A) is not correct, but (R) is correct.
A.
Both (A) and (R) are correct, (R) is the correct explanation of (A).
The -NH2 group of aniline is a very strong electron donor (+M effect), hence it activates the benzene ring thoroughly and the electrophilic aromatic substitutions on the benzene ring are very easy to take place at ortho and para-positions.
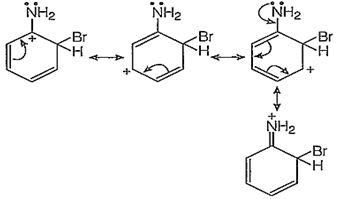
o-bromination:
p-bromination :
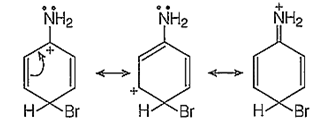
In addition to the usual resonating structures, that stabilizes the intermediate carbonium ion, the resonating strucutres formed by the interaction of lone pair electrons of nitrogen with the positively charged carbon of the ring also increase the stability of the carbonium ion formed durig the atttack of Br+ ion (electrophile) on o- and p-positions.
In which of the following properties, the two enantiomers of lactic acid differ from each other?
Sign of specific rotation
Density
Melting point
Refractive index
