 Multiple Choice Questions
Multiple Choice QuestionsWith what velocity should an -particle travel towards the nucleus of a copper atom to arrive at a distance of 10-13m from the nucleus of the copper atom?(K = 9x 109 Nm2/C2 (Mass of -particle = 6.64 x 10-27kg).
634 x 106 ms-1
634 x105 ms-1
5.34 x 106 ms-1
534 x 105 ms-1
What is the ratio of the velocities of CH4 and O2 molecules so that they are associated with de-Broglie waves of equal wavelengths?
1:2
2: 1
3: 2
1: 2
Nitrogen forms stable N2 molecule, but phosphorus is converted to P4 from P2 because
bonding is strong in phosphorus
bonding is weak in phosphorus
double bond is present in phosphorus
single P-P bond is weaker than N-N bond
An amorphous solid (X) burns in air to form a gas (Y) which turns lime water milky. This gas decolarises aqueous solution of acidified KMnO4 gas (Y)reacts with oxygen to give another gas (Z) which is responsible for acid rain. X, Y and Z are
| X | Y | Z |
| C | CO | CO2 |
| X | Y | Z |
| S | SO2 | SO3 |
| X | Y | Z |
| P | P2O3 | P2O5 |
| X | Y | Z |
| S | SO3 | H2SO4 |
A black power when heated with conc. HCl gives a greenish yellow gas. The gas acts as an oxidising and a bleaching agent. When it is passed are slaked lime, a white power is formed which is a ready source of gas. The black powder and white power respectively are
KClO3 and NaClO3
MnO2 and CaCOCl2
MnO2 and KClO3
MnCl4 and COCl2
Which of the following is an organometallic compound?
Lithium methoxide
Lithium acetate
Lithium dimethylamide
Methyl lithium
The major product obtained in the reaction


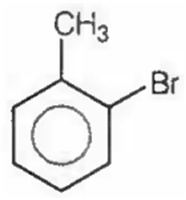


D.

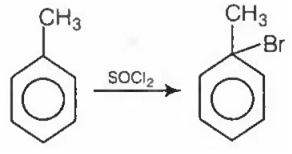
Ease of substitution of hydrogen is 3° >2°> 1 °.
