 Multiple Choice Questions
Multiple Choice QuestionsThe product of uncertainty in velocity and uncertainty in position of a micro particle of mass 'm' is not less than in which of the following?
h ×
× m
An element has [Ar] 3d1 configuration in its +2 oxidation state. Its position in the periodic table is
period - 3, group - 3
penod - 3, group - 7
period - 4, group - 3
period - 3, group - 9
In which of the following molecules, maximum number of lone pairs is present on the central atom?
NH3
H2O
ClF3
XeF2
D.
XeF2
Among the given pair of molecules, maximum number of lone pairs are present in XeF2.
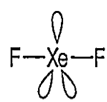
Which one of the following is the kinetic energy of a gaseous mixture containing 3g of hydrogen and 80g of oxygen at temperature T (K)?
3 RT
6 RT
4 RT
8 RT
A, B, C and D are four different gases with critical temperatures 304.1, 154.3, 405.5 and 126.0 K respectively. While cooling the gas, which gets liquefied first?
B
A
D
C
40 mL of x M KMnO4 solution is required to react completely with 200 mL of 0.02 M oxalic acid solution in acidic medium. The value of x is
0.04
0.01
0.03
0.02
Given that,
C (s) + O2 (g) → CO2 (g) ; = -x kJ mol-1
2CO (g) + O2 (g) → 2CO2 (g) ; = -y kJ mol-1
The enthalpy of formation of CO will be
At 400 K, in a 1.0 L vessel, N2O is allowed to attain equilibrium, N2O4 (g) 2NO2 (g)
At equilibrium, the total pressure is 600 mm Hg, when 20% of N2O4 is dissociated. The value of KP for the reaction is
50
100
150
200
In which of the following salts, only cationic hydrolysis is involved?
CH3COONH4
CH3COONa
NH4Cl
Na2SO4
