 Multiple Choice Questions
Multiple Choice QuestionsThe oxidation states of Cr in [Cr(H2O)6]Cl3,[Cr(C6H6)2], and K2[Cr(CN)2 (O)2(O2)(NH3)] respectively are :
+3, +2, and +4
+3, 0, and +6
+3, 0, and +4
+3, +4, and +6
Which of the following compounds will be suitable for Kjeldah1’s method for nitrogen estimation?

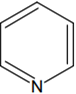


D.

Kjeldahl's method is used for Aniline. This method is used for quantitative analysis of N compound in organic substance (NH3/NH4+).
This method is not used in the case of nitro, azo compounds and also to the compounds containing nitrogen in the ring e.g. Pyridine.
The increasing order of basicity of the following compounds is:

(d) < (b) < (a) < (c)
(a) < (b) < (c) < (d)
(b) < (a) < (c) < (d)
(b) < (a) < (d) < (c)
Phenol reacts with methyl chloroformate in the presence of NaOH to form product A. A reacts with Br2 to form product B. A and B are respectively:




Phenol on treatment with CO2 in the presence of NaOH followed by acidification produces compound X as the major product. X on treatment with (CH3CO)2O in the presence of catalytic amount of H2SO4 produces:




The compound that does not produce nitrogen gas by the thermal decomposition is
(NH4)2SO4
Ba(N3)2
(NH4)2Cr2O7
NH4NO2
Which of the following salts is the most basic in aqueous solution?
Pb(CH3COO)2
Al(CN)3
CH3COOK
FeCl3
