 Multiple Choice Questions
Multiple Choice QuestionsH2S gas when passed through a solution of cations containing HCl precipitates the cations of second group of qualitative analysis but not those belonging to the fourth group. It is because:
presence of HCl decreases the sulphide ion concentration
presence of HCl increases the sulphide ion concentration
solubility product of group II sulphides is more than that of group IV sulphides
sulphides of group IV cations are unstable in HCl
Which one of the following oxides is expected to exhibit paramagnetic behaviour?
CO2
SO2
ClO2
SiO2
A solution has a 1 : 4 mole ratio of pentane to hexane. The vapour pressure of the pure hydrocarbons at 20C are 440 mm of Hg for pentane and 120 mm of Hg for hexane. The mole fraction of pentane in the vapour phase would be
0.549
0.200
0. 786
0.478
4.5 g of aluminium (Atomic mass 27 amu) is deposited at cathode from Al3+ solution by a certain quantity of electric charge. The volume of hydrogen produced at STP from H+ ions in solution by the same quantity of electric charge will be
22.4 L
44.8 L
5.6 L
11.2 L
Which among the following is the most stable carbocation?
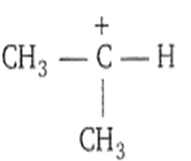
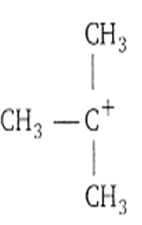
CH3H2
B.
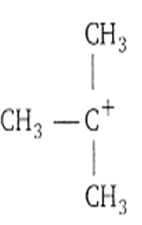
The most stable carbocation is t-alkyl carbocation because the order of stability of alkyl carbocation is
t-alkyl > s-alkyl > p-alkyl > CH carbocation
This stability order is described with the help of hyperconjugation and inductive effect.
Products of the following reaction
CH3C C.CH2CH3 .... are
CH3CHO + CH3CH2CHO
CH3COOH + CH3COCH3
CH3COOH + HOOC.CH2CH3
CH3COOH + CO2
Which one of the following alkenes will react faster with H2 under catalytic hydrogenation conditions?
(R = Alkyl substituent)
![]()
![]()


Which one of the following is expected to exhibit optical isomerism? (en = ethylenediamine)
cis-[Pt (NH3)2Cl2]
trans-[Co (en)2 Cl2]
trans- [Pt (NH3)2Cl2]
cis-[Co(en)2Cl2]
