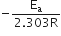Activation energy. Excess of energy which must be supplied to reactant to undergo the chemical reaction called activation energy.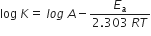
where, K = rate constant
A = Arrhenius constant
Ea = Activation energy
R = Gas constant
T = Temperature (K)
The slope of the line is equal to