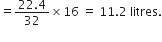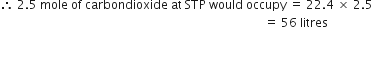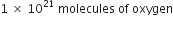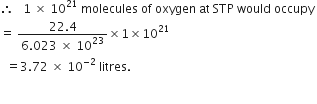Calculate the volume at STP occupied by:
(i) 16 g of oxygen
(ii) 2·5 moles of CO2
(iii) 1 × 1021 molecules of oxygen.
(i) 16 g of oxygen.Gram molecular mass of oxygen = 32 g
Now 32 g oxygen at STP occupies volume = 22.4 litres
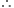
16 g oxygen at STP would occupy volume
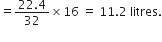
(ii) 2.5 moles of CO
2
1 mole of carbondioxide at STP occupies = 22.4 litres
(iii)
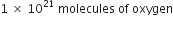

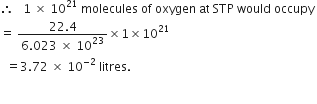
228 Views