Discuss in brief sp3 hybridisation. Explain the formation of methane and ethane.
sp3 hybridisation: Electronic configuration of carbon (Z = 6) in the excited state is 1s2 2s1

This type of hybridization involves the mixing of all four filled orbitals i.e. 1s and 3p orbitals to form four new orbitals called sp3 hybrid orbitals of equivalent enthalpies and identical shapes.
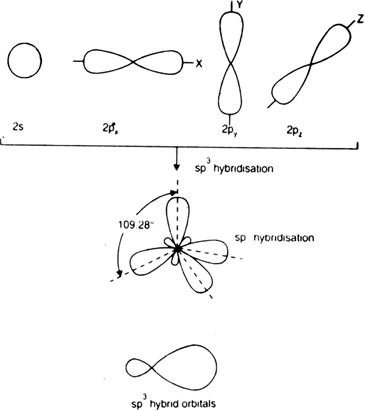
Fig. Representation of sp3 hybridization:
A single sp3 hybrid orbital.
The four sp3 hybrid orbitals are directed towards the four corners of a tetrahedral. The angle between two adjacent sp3 hybrid orbitals. Each sp3 hybrid orbital has 1/4 s-character and 3/4 p-character. sp3 hybridization is also known as tetrahedral hybridisation.
(i) The molecular orbital structure of methane: In methane molecule, carbon atom undergoes sp3 hybridisation. Each sp3 hybrid orbital overlaps with 1s orbital of hydrogen atom along the internuclear axis to form four σ bond.

Molecular orbital structure of methane
The four C – H bonds are directed towards the four corners of a regular tetrahedron. So methane has a tetrahedral structure. Each H – C – H bond angle is of 109°.28’. Each C – H bond length is 109 pm (1.09 Å).
(ii) Molecular orbital picture of ethane: In ethane molecule, both carbon atoms are in the sp3 hybrid state. In its formation, one hybrid orbital of one carbon atom overlaps with one sp3 hybrid orbital of a second carbon atom along the internuclear axis to form a sigma (σ) C – C bond. The remaining three sp3 hybrid orbitals of each carbon atom overlap with 1 s orbital of hydrogen atom axially to form six sigma C – H bonds.

The length of C-C bond in ethane is 154 pm (or 1- 54 Å) and that of each C - H bond is 109 pm (or 1-09 Å).
1378 Views




